Anode is Positive or Negative
As the anode half cell becomes overwhelmed with Cu 2 ions the negative anion of the salt will enter the solution and stabilized the charge. In order to balance the charge on both sides of the cell the half cells are connected by a salt bridge.
Components of Cells and Batteries.

. Whereas in an electrolytic cell it is given as positive. Silicon is a promising alternative to lithium as an anode for Li-S batteries due to the high theoretical capacity of 3580 mAh g 1 Li 15 Si 4As is known the practical application of Si-based anodes is limited by a serious loss of capacity which is attributed to mechanical. The larger plate belongs to the negative cathode lead.
Prepare cells with different electrodes and concentrations and measure their voltages. In one form of electroplating the metal to be plated is located at the anode of the. The movement of the lithium ions creates free electrons in the.
Negatively charged ions are atoms with one electron too many. It acts as an electron donor. Eine Anode entspricht somit einem Elektronenakzeptor.
Pouch cells in this study used a single-crystal LiNi 05 Mn 03 Co 02 O 2 94 active 16 mg cm 2 35 g cm 3 positive electrode facing a bare copper current collector as the negative. An anode ray also positive ray or canal ray is a beam of positive ions that is created by certain types of gas-discharge tubesThey were first observed in Crookes tubes during experiments by the German scientist Eugen Goldstein in 1886. The anode and cathode store the lithium.
Thomson led to the development of mass spectrometry. The anode acts as a negatively charged terminal in the galvanic cell or in the discharge battery as the current flows into the cell through the cathode. Cathode - The electrode that receives electrons - positive ions are discharged negative ions are formed.
A cathode is a negative side. Due to the polarization reaction of the electrode the anode peak shifts to 167 V and 189 V in the second third and fourth cycles which can be attributed to the oxidation of. Its usually made of a metal that oxidizes and sends electrons to the cathode the positive electrode.
Falling positive anode current accompanied by rising anode voltage gives the anode characteristic a region of negative slope and this corresponds to a negative resistance which can cause instability in certain circuits. An anode is a negative electrode and its one of the essential parts of a battery. The Anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during and electrochemical reaction.
The anode is the electrode where electricity moves into. Return Current Path - The metallic pathway connecting the anode to the cathode. The reverse is true for electrolytic cells where the anode is positive and the cathode is negative.
The current that is flowing inward. A battery is made up of an anode cathode separator electrolyte and two current collectors positive and negative. In addition an anode can be a wire or a plate having an excess positive charge.
Eine Anode von griechisch ἄνοδος ánodos Aufstieg wörtlich Weg nach oben ist eine Elektrode die beispielsweise aus einem Vakuum freie Elektronen aufnimmt oder aus einem Elektrolyten unter Elektronenaufnahme Anionen entlädt oder Kationen erzeugt also Oxidationsreaktionen stattfinden lässt. When they reach the positive anode they transfer their electrons to it and lose their negative charge. A reaction occurs at each.
Cells are comprised of 3 essential components. In the subsequent anode scanning the anode peak in the first cycle is 161 V and 181 V. Krzysztof Jan Siczek in Next-Generation Batteries with Sulfur Cathodes 2019.
The smaller plate indicates the positive anode lead. In a Galvanic cell the anode is negative and the cathode positive. The Anode and Cathode.
The electrolyte carries positively charged lithium ions from the anode to the cathode and vice versa through the separator. Unfortunately some jumbo LEDS have the plates reversed so this is not a fool-proof method. Thus harsh conditions were explored with cathode mass loading up to 7 mg cm 2 and NP down to 12 fig.
The anode is usually the positive side. The cathode is the electrode where electricity is given out or flows out. The Cathode is the positive or oxidizing electrode that acquires electrons from the external circuit and is reduced during the electrochemical reaction.
In a higher range of anode voltage the anode voltage sufficiently exceeds that of the screen for an increasing proportion of. Later work on anode rays by Wilhelm Wien and J. At the same time negatively charged ions move to the positive electrode called the anode.
This is an electrochemical reaction that produces electrons ie electricity. The cathode is protected from corrosion. In an electrolytic cell oxidation reaction takes place at the anode.
It acts as an electron acceptor. Electrolyte - The conductor through which current is carried. If the LED has a flat area on the plastic housing the lead adjacent to the flat area is the negative cathode lead.
Considering practical implementation it is necessary to use a high areal cathode capacity and limited Na metal namely low negative to positive electrode capacity NP 2 but it is largely neglected in previous work table S7. Electrolytes include aqueous solutions or other liquids. Similarly in the cathode half cell as the solution becomes more negatively charged cations from.

Anodes And Cathodes Positive And Negative Bar Chart Mcat
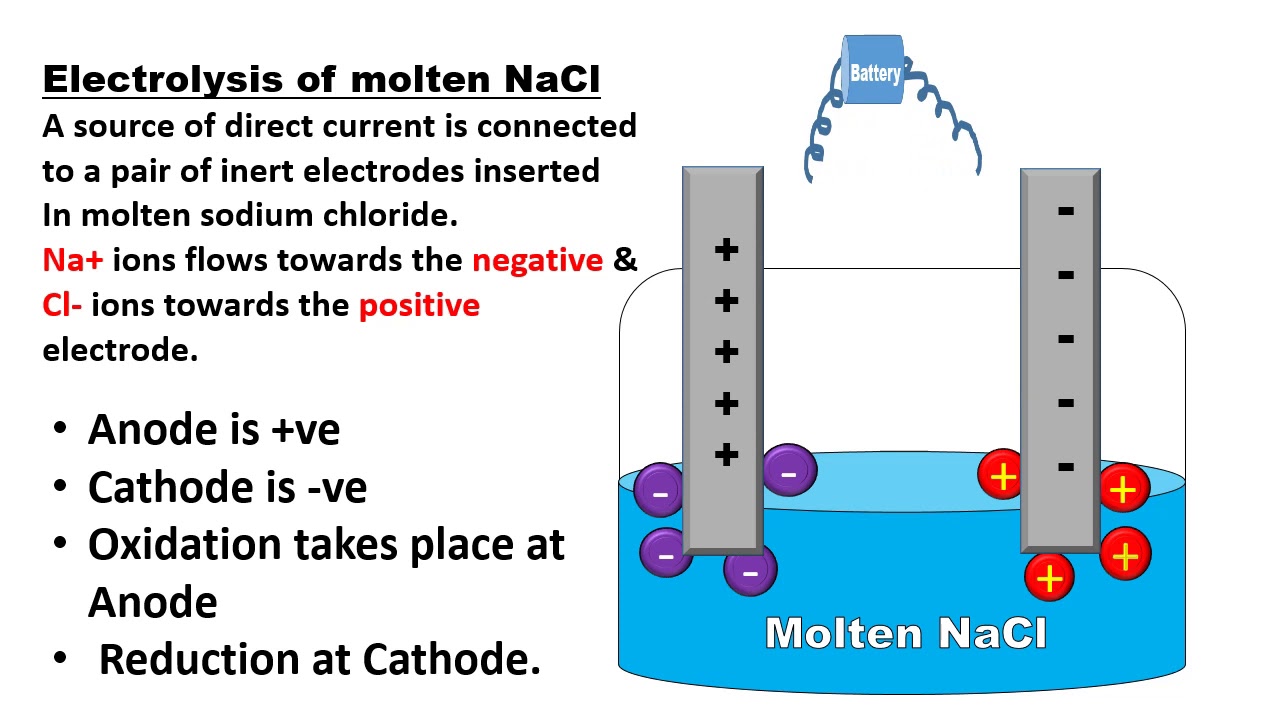
What Is Electrolytic Cell Electrochemistry Chemistry Basics Chemistry

Electrophoresis Chemistry Anode Cathode Diagram Chemistry Respiratory Therapy Study Materials

How To Find Anode And Cathode In Diode Diode Black Rings Tutorial
Comments
Post a Comment